
Because of their low reactivity, we often use them to make coins, jewelry, etc., so we call them “noble metals.” Examples include copper, gold, silver, etc. They are relatively very stable and do not form compounds readily. These metals are thus called “active metals.”Īt the bottom of the series, there are transition metals. Moreover, they easily react to form compounds. These are more reactive and easily undergo oxidation than metals at the bottom.
#Reactivity of metals series#
Another common name for this series is “ activity series.” Furthermore, this series lists metals in order of decreasing reactivity.Īt the top of the series, it has alkali metals and alkaline earth metals.

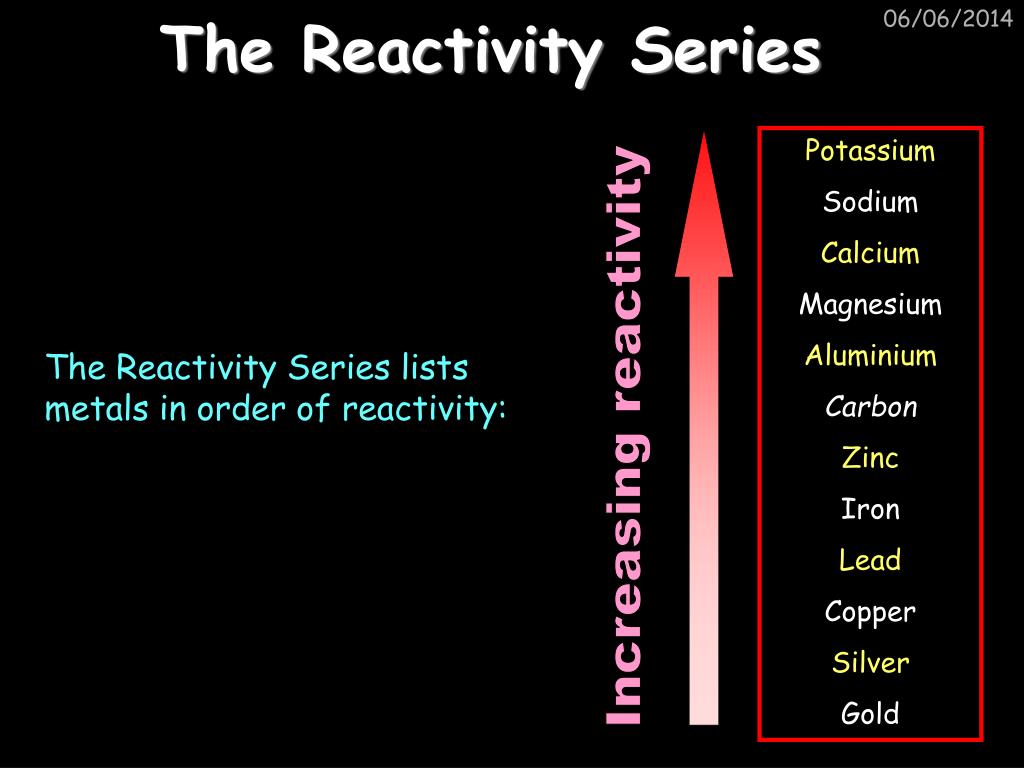
It provides enough information about the relative reactivity of metals in aqueous solutions under standard conditions.
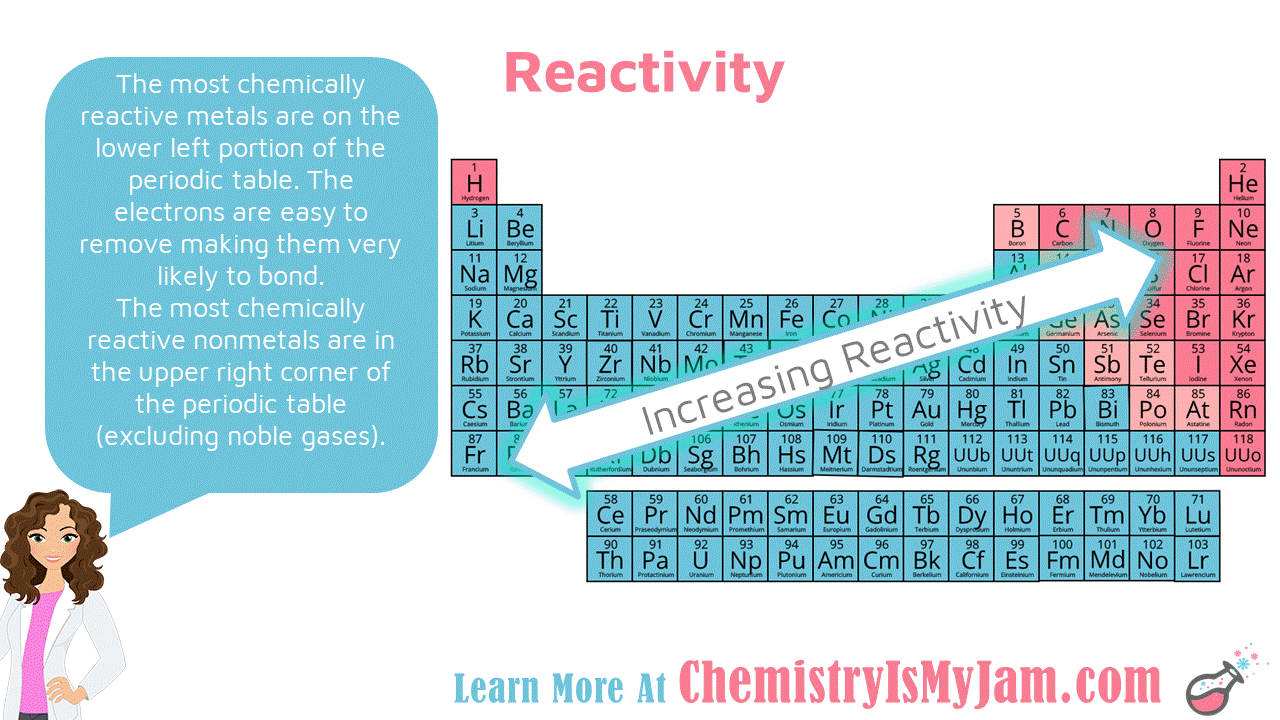
Summary – Electrochemical Series vs Reactivity Series What is Electrochemical Series?Įlectrochemical series is a list of chemical elements that shows the order of their standard electrode potentials. Electrochemical Series vs Reactivity Series in Tabular Formĥ. The key difference between electrochemical series and reactivity series is that electrochemical series gives the order of the standard electrode potentials, whereas reactivity series gives the arrangement of metals in the descending order of the reactivity of those metals.Įlectrochemical series and reactivity series are important listings of chemical elements electrochemical series includes chemical elements with electrode potentials, while reactivity series includes metals.


 0 kommentar(er)
0 kommentar(er)
